Enabled by clonal-level microbiome profiling engraftment analysis, metabolomics and multi-omics analysis
The Microbiome & Probiotics R&D and Business Collaboration Forum recently hosted Marianne Koliana, VP of Business Development at Clinical Microbiomics. In the presentation she delved into crucial aspects of clinical trials, including regulatory compliance, engraftment analysis, and the pivotal role of multi-omics approaches, offering a comprehensive understanding of the evolving field.
Regulatory Compliance and Quality Assurance in Clinical Trials
The increasing development of microbial therapeutics and probiotics for patient treatment draws more attention. Authorities are focusing on the quality of data, prompting the need for submission-ready data packages. Marianne emphasized, “it’s extremely important that everything we generate is according to a qualified platform and is ready for submission.“. Clinical Microbiome, recognizing this, embarked over the last few years to bridge the gap between scientific innovation and regulatory demands.
She detailed a comprehensive regulatory compliance approach throughout the pipeline. From study planning to reporting, and the steps between collection and storage, extraction, data generation, and data analysis. The challenge, she noted, lies in data analysis. The presentation explored various aspects of this, such as the gene catalogue, the scripts applied, the process of data cleaning and the validation of applications and programs.
Acknowledging complexity, Marianne stated without hindering science they must start to focus on coupling the science with quality. Ensuring innovative research doesn’t stop due to a lack of qualified platforms.

Engraftment Analysis for Therapeutic Success
Robust microbiome profiling is foundational to a successful microbiome study. Marianne highlighted developments since 2014 and then delved into engraftment analysis. She defined engraftment as the intentional incorporation of new organisms into a host and restoration as re-establishing something that was destroyed. This distinction is pivotal as clinical trials often involve antibiotics, which deplete the microbiome present in the individual.
Regarding the slide, Marianne explained, “Strains here refer to cultured strains, and are here shown with an NCBI accession number. However, for natural populations each person possesses a unique clonal population. People in the audience will have different, you can say personal Akkermansia muciniphila populations. Distinguishing between the recipient Akkermansia muciniphila population and a probiotic Akkermansia muciniphila strain is crucial for an engraftment analysis of a probiotic. The same goes for FMT administration. This requires de novo profiling of strains and mapping against reference genomes isn’t sufficient for this distinction.“
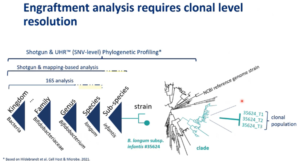
Marianne emphasized the need for technologies capable of differentiation and provided insights into clonal-level microbiome profiling. She described the importance of identifying single-nucleotide polymorphic positions between samples to enable Engraftment tracking. With Clinical Microbiomics’ technology, it becomes possible to identify species and strains associated with higher engraftment rates and potentially better clinical outcomes. She also described how Clonal-level resolution empowers researchers to identify species and strains linked to successful engraftment, enriching our comprehension of therapeutic efficacy.
Unlocking Mechanisms with Multi-Omics analysis:
Microbiome analysis has evolved beyond just taxonomical composition. The presentation stressed the value of integrating multi-omics layers to understand the interplay between microbial communities and human health. Leveraging metabolomics and other omics data, this perspective offers an understanding of microbial activities and their impact on health.
By merging diverse omics datasets, researchers gain insights that go beyond taxonomy. Marianne cited the power of these tools to delve into genes, metabolite production, and overall data integration. Multi-omics analysis is pivotal for deciphering mechanisms-of-action and uncovering correlations with clinical responses.
In conclusion, Marianne’s presentation illuminated the dynamic landscape of microbial therapeutics. Regulatory compliance, engraftment analysis, and multi-omics integration form a triad that propel the field forward. As the field of microbial therapeutics continues to expand, the synergy between scientific innovation, rigorous quality standards, and multi-omics insights holds the key to translating research into impactful clinical applications.
 Marianne Koliana, VP, Business Development, Clinical Microbiomics
Marianne Koliana, VP, Business Development, Clinical Microbiomics
This article was written by Ellie Egginton summarising the presentation given by Marianne Koliana.





