Introduction
BIOASTER, a not-for-profit research institute based in France, is dedicated to microbiology and infectious diseases. The institute’s primary mission is to collaborate with academic and industrial partners to develop and advance their innovations, starting from the idea towards the final product. The institute’s work revolves around addressing crucial biological questions and seeking solutions through various technological units. The Microbiota Program of BIOASTER focuses on exploring the role of the microbiota in the health state of the host.
The Role of Intestinal Epithelial Barrier in Host – Microbiota Interactions
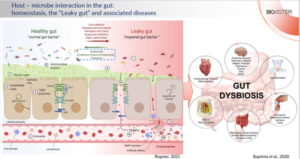
The intestinal microbiota plays a key role in human health, while the gut epithelium maintains the necessary physical barrier between the microbes and the internal organs. Healthy gut epithelium produces mucus and a variety of anti-microbial factors thus shaping the intestinal microbiota and allowing the balance between the host immune response and the gut microbes. Disruption of the epithelial barrier balance provokes a leakage of microbes and their components beyond epithelium, activating local immune system and inducing inflammation. When it becomes chronic, uncontrolled activation of immune system leads to a variety of inflammatory, metabolic, and neurodegenerative pathologies (Figure 1).
In-vitro and Ex-vivo Evaluation of Pre and Probiotics
Probiotics and prebiotics are being extensively developed to aid maintaining and repairing the compromised balance between the intestinal microbiota and the host immune response. Their selection and validation is a complex process involving in-vitro and in-vivo models. While the former is lacking the complexity of the physiological system, the latter often fails to reflect the true effect in humans. Therefore, BIOASTER developed a two-step ex-vivo platform involving both human fecal microbiota and/or primary immune cells separated by an intestinal epithelial barrier for evaluation of prebiotics and probiotics (Fig. 2). 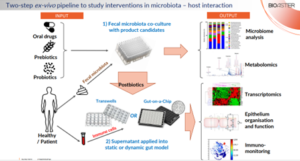
First, fecal microbiota from human donors is co-cultured with probiotics or used for fermentation of prebiotics. At this step the effect of the studied product candidates on microbiome and metabolome can be explored. Then, supernatant and/or heat-inactivated bacteria (also called postbiotics) are employing in a static (Transwell-based) or dynamic (microfluidics-based “Gut-on-a-Chip”) gut model, that includes epithelial layer and human primary immune cells. These models allow to investigate the impact of the studied pre- and probiotics on epithelial barrier function, host cell transcriptomics and immune system activation.
Example Project: Evaluating Prebiotics Impact on Epithelial Homeostasis
Employing three fecal microbiota sample from healthy donors, researchers fermented 15 prebiotics (synthetic polysaccharides) and tested the impact of the generated supernatants on epithelial homeostasis by assessing the barrier function of epithelial cell grown on filters of Transwells as well as their gene transcription (Figure 3). Analysis of epithelial barrier function (using Dextran-Fluorescein (FITC) permeability) appeared inconclusive, attributed to anticipated donor variations, as the same prebiotics could have opposite effect depending on the microbiota origin. 
However, crossing these results with epithelial gene expression profiles unearthed intriguing pattern linking it with barrier function. It appeared that the reinforcement of the barrier function occurred only when incubation of fermented prebiotics with epithelial cells resulted in downregulation of Claudin-2 and matrix metalloproteinase-7 (MMP7), mirrored by an increase in E-Cadherin expression.
Diving into the mechanism, the strength of the tight junctions is regulated in intestinal epithelial cells by a balance between “leaky” and “tight” members of the Claudin family. Claudin-2 leans toward a “leaky” nature, while Claudin-4 embodies a “tight” attribute. Therefore, a drop in Claudin-2 shifts the balance in a favor of “tight” Claudins thus contributing to stronger bonds between the cells. E-Cadherin’s role as an adjacent junction protein is well known, where more is merrier. Additionally, reduction in MMP7, a metalloproteinase degrading extracellular matrix and junction proteins (including E-cadherin), further contributes to epithelial integrity.
Example Project: Investigating Oral Probiotics in SARS-CoV-2 Infection
Beyond its impact on intestinal health, gut microbiota has an effect on distant organs. This is the case of the so-called “Gut-lung axis”, where the immune response in the lungs is targeted by orally delivered pre and probiotics. In the context of the recent Covid-19 pandemics, researchers in collaboration with Pileje Laboratoire investigated the potential use of oral probiotics for treatment and/or prophylaxis of SARS-CoV-2 infection. For this end, probiotic strain Lacticaseibacillus rhamnosus LA 801 was added on top of epithelial cells grown on filters of Transwell, while primary leukocytes from human peripheral blood where incubated beneath the filter. In addition, the leukocytes were exposed to human lung cell lines infected with SARS-CoV-2 and the impact on immune cell activation and cytokine secretion was assessed.
Focusing on surface activation markers of innate cell, researchers detected activation of Natural Killer (NK) cells, but not of Monocytes, in response to virus-infected lung cells while no impact of probiotics was detected. Nevertheless, evaluation of secreted cytokines revealed a significant increase of pro-inflammatory cytokines IL-1β and TNFα (and a trend in IL-6) production in response to the probiotics. Subsequent analysis of intracellular cytokines production revealed a strong accumulation of IL-1β and TNFα in Monocytes upon exposure to infected lung cells suggesting an important role of these cells in the observed response to SARS-CoV-2 (Figure 4). 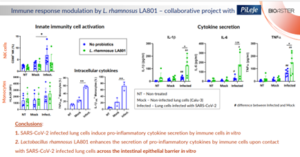
Thus, given its immuno-stimulatory effect, L. rhamnosus LA 801 can be considered for prophylaxis of Covid-19 and/or treatment of the very early stages of the infection when an enhancement of the anti-viral immune response is desired.
Example Project: Immunocompetent Mid-Throughput “Gut-on-a-Chip” Model
The last project is the set-up of a mid-throughput “Gut-on-a-Chip” model using commercially available microfluidic plate containing 40 chips. Each chip features three channels while the central one is filled with extracellular matrix, most often collagen, thus separating between the other two (Figure 5).  Each channel is connected to a small reservoir at its every end, and, thanks to a rocking movement, a flow of the liquid (medium) is generated in the channels. Epithelial cells are seeded in the upper channel and, thanks to a shear stress created by the medium flow, a leak-tight epithelial monolayer in the form of a hollow tubule is generated after only 4 days of incubation. Then, human peripheral blood mononuclear cells (PBMCs) are added to the lower channel while E. coli LPS or fecal supernatant (also called fecal water) is added to the upper channel with epithelial cells to mimic the gut environment. These treatments resulted in impairment of the epithelial barrier (assessed by Fluorescein paracellular permeability and by trans-epithelial electrical resistance (TEER)), secretion of pro-inflammatory cytokine IL-8, activation of Monocytes and disorganization of E-cadherin spatial expression. Of note, these effects were not observed in the absence of PBMCs thus emphasizing the key role of immune cells in this model of intestinal inflammation.
Each channel is connected to a small reservoir at its every end, and, thanks to a rocking movement, a flow of the liquid (medium) is generated in the channels. Epithelial cells are seeded in the upper channel and, thanks to a shear stress created by the medium flow, a leak-tight epithelial monolayer in the form of a hollow tubule is generated after only 4 days of incubation. Then, human peripheral blood mononuclear cells (PBMCs) are added to the lower channel while E. coli LPS or fecal supernatant (also called fecal water) is added to the upper channel with epithelial cells to mimic the gut environment. These treatments resulted in impairment of the epithelial barrier (assessed by Fluorescein paracellular permeability and by trans-epithelial electrical resistance (TEER)), secretion of pro-inflammatory cytokine IL-8, activation of Monocytes and disorganization of E-cadherin spatial expression. Of note, these effects were not observed in the absence of PBMCs thus emphasizing the key role of immune cells in this model of intestinal inflammation.
In summary, this experimental system allows a mid-throughput screening of different product candidates for their impact on both epithelial and immune cells in a model mimicking intestinal environment.
Conclusion
Through its Microbiota Program, BIOASTER remains at the forefront of exploring the critical role of the epithelial barrier in host-microbiota interactions. Their innovative ex-vivo and in-vitro models provide valuable platforms for evaluating prebiotics and probiotics, fostering advancements in microbiology and infectious disease research.

Ilia Belotserkovsky, Scientific Project Manager, Bioaster






